
6 Ways Kratom BDNF Effects Could Transform Brain Health

Faust
The human brain relies on a key protein called brain-derived neurotrophic factor, or BDNF (a molecule that supports neuron survival, growth, and synaptic plasticity). This protein plays a central role in learning, memory, mood regulation, and overall cognitive function. Kratom, derived from the leaves of the Mitragyna speciosa tree native to Southeast Asia, contains alkaloids that interact with opioid and other receptors in the body. A growing question among researchers and users is whether kratom BDNF effects exist in humans and, if so, how they might influence brain health.
Direct evidence from controlled human trials remains limited, but indirect clues from pharmacology, animal research, and user reports provide a foundation for informed discussion. This blog examines six potential pathways through which kratom might interact with BDNF systems. Each section focuses on mechanisms grounded in established science, while acknowledging gaps in human-specific data.
What Is BDNF and Why Does It Matter?
BDNF is a neurotrophin, a family of proteins that regulate neuron development and maintenance. First isolated in 1982, it is expressed throughout the central nervous system, with highest concentrations in the hippocampus and cortex, regions critical for memory and executive function. BDNF binds to TrkB receptors on neuron surfaces, triggering signaling cascades that promote cell survival, dendritic branching, and long-term potentiation, the cellular basis of learning.
Circulating BDNF levels respond to external factors. Aerobic exercise increases BDNF expression within hours, while chronic stress or sleep deprivation reduces it. Low BDNF correlates with impaired cognitive performance and increased risk for mood disorders. In clinical settings, serum BDNF is used as a biomarker for brain health. Understanding any substance’s impact on this protein therefore carries implications for mental resilience and adaptability.
Kratom enters this picture through its primary alkaloids, mitragynine and 7-hydroxymitragynine, which cross the blood-brain barrier and modulate multiple neurotransmitter systems. The question of kratom BDNF effects hinges on whether these interactions create conditions that either enhance or suppress neurotrophin activity.
How Kratom Interacts With the Central Nervous System
Primary Receptor Targets
Kratom alkaloids primarily target mu-opioid receptors, but they also influence serotonin, dopamine, adrenergic, and cannabinoid pathways. At lower doses (1-5 grams of leaf), mitragynine acts as a partial agonist, producing mild stimulation and analgesia. Higher doses (5-15 grams) shift toward sedation via stronger opioid receptor engagement.
Dose-Dependent Effects on BDNF
These dose-dependent effects matter because BDNF expression is sensitive to receptor signaling. For example, balanced serotonin activity supports hippocampal BDNF, whereas excessive opioid stimulation can downregulate it in animal models. Kratom’s unique profile, combining opioid-like action with adrenergic stimulation, creates a complex neurochemical environment that may indirectly shape BDNF dynamics.
Pharmacokinetics and Bioavailability
Bioavailability also plays a role. Oral consumption yields peak plasma levels of mitragynine within 1-2 hours, with a half-life of about 3-4 hours. This pharmacokinetic profile allows sustained but moderate receptor occupancy, potentially avoiding the intense fluctuations that disrupt neurotrophin balance.

Pathway 1: Reducing Withdrawal-Related Stress on Neural Circuits
Individuals transitioning from opioid dependence often experience cognitive deficits during withdrawal, including poor concentration and memory lapses. These symptoms partly reflect suppressed BDNF activity caused by elevated cortisol and dynorphin.
Kratom is frequently used in this context to manage withdrawal discomfort. By providing partial opioid receptor agonism, it stabilizes mood and reduces physical symptoms without the intensity of full agonists. This smoother transition may limit stress-induced BDNF suppression. Preclinical data show that mitigating withdrawal stress preserves hippocampal neurogenesis, a process BDNF directly supports.
In practical terms, users who taper opioids with kratom report fewer “brain fog” episodes compared to cold-turkey cessation. While human BDNF measurements are absent, the mechanism aligns with known stress-neurotrophin interactions.
Pathway 2: Modulating Mood Via Serotonin and Dopamine Balance
Mood stability influences BDNF expression through the prefrontal cortex and limbic system. Selective serotonin reuptake inhibitors (SSRIs) increase BDNF in depressed patients within weeks, correlating with symptom improvement.
Kratom inhibits serotonin and norepinephrine reuptake at certain doses, resembling atypical antidepressants. This action may create a favourable environment for BDNF transcription. Animal studies of mitragynine demonstrate antidepressant-like effects in forced swim tests, accompanied by elevated hippocampal BDNF mRNA, though species differences limit direct extrapolation. For humans, consistent low-dose kratom use is associated with improved emotional regulation in anecdotal reports. If this reflects sustained monoamine balance, it could support BDNF-mediated plasticity over time.
Pathway 3: Enhancing Alertness and Cognitive Processing Speed
Sustained attention relies on prefrontal BDNF, which strengthens working memory circuits. Stimulants like methylphenidate increase BDNF in attention-related regions, improving task performance.
Low-dose kratom produces wakefulness and focus without the cardiovascular strain of traditional stimulants. This effect stems from alpha-2 adrenergic antagonism and dopamine release in the nucleus accumbens. By maintaining moderate arousal, kratom may avoid the post-stimulus BDNF crash seen with amphetamine-like compounds. Users describe enhanced productivity during detailed work, suggesting possible support for attention networks. Direct BDNF assays would be needed to confirm this pathway in humans.
Pathway 4: Protecting Memory Consolidation During Sleep
Sleep is a major driver of BDNF release, particularly during slow-wave phases when memory traces are consolidated. Opioid agonists generally suppress REM sleep, but kratom’s partial agonism appears to preserve sleep architecture at moderate doses. Survey data indicate that evening kratom use (red-vein strains) promotes restful sleep without morning grogginess.
If sleep quality remains intact, nightly BDNF surges can proceed unimpeded, supporting long-term memory storage. This pathway is especially relevant for shift workers or students using kratom to manage fatigue. Preserved sleep-BDNF cycles could counteract cognitive wear from irregular schedules.

Pathway 5: Mitigating Chronic Stress Through HPA Axis Regulation
The hypothalamic-pituitary-adrenal (HPA) axis governs stress responses, and dysregulation lowers BDNF via glucocorticoid receptor activation. Adaptogens like ashwagandha restore HPA balance and raise BDNF in stressed populations. Kratom’s analgesic and anxiolytic properties may dampen perceived stress, reducing cortisol output. Rodent studies show mitragynine attenuates stress-induced corticosterone spikes, preserving BDNF in the prefrontal cortex. For individuals with high occupational stress, this buffering effect could maintain neurotrophin levels, supporting decision-making and emotional resilience.
Pathway 6: Supporting Social Cognition and Empathy Networks
Social interaction activates BDNF in the amygdala and insula, areas involved in emotional recognition. Isolation reduces BDNF, while enriched environments reverse the decline. Kratom’s mood-elevating effects at social doses (2-4 grams) may facilitate engagement without intoxication. Users report easier conversations and reduced social anxiety, potentially allowing BDNF-dependent plasticity in empathy circuits. This pathway remains speculative but aligns with observations of improved interpersonal function in controlled settings.
Current Limitations and Research Gaps
- No human BDNF data: No published trials have measured serum or CSF BDNF levels before and after kratom use.
- Extrapolation only: Current evidence relies solely on receptor pharmacology, animal models, and self-reported outcomes.
- High variability: Strain potency, preparation method, and individual metabolism create significant noise in results.
- Safety unknowns: Tolerance, dependence risk, and drug interactions remain poorly quantified in humans.
- No regulatory approval: The FDA has not approved kratom for any medical use.
- Quality inconsistency: Commercial products show wide variation in alkaloid content and purity.
Regulatory Status and Quality Control Standards Worldwide
Kratom in Canada: Legal but Regulated
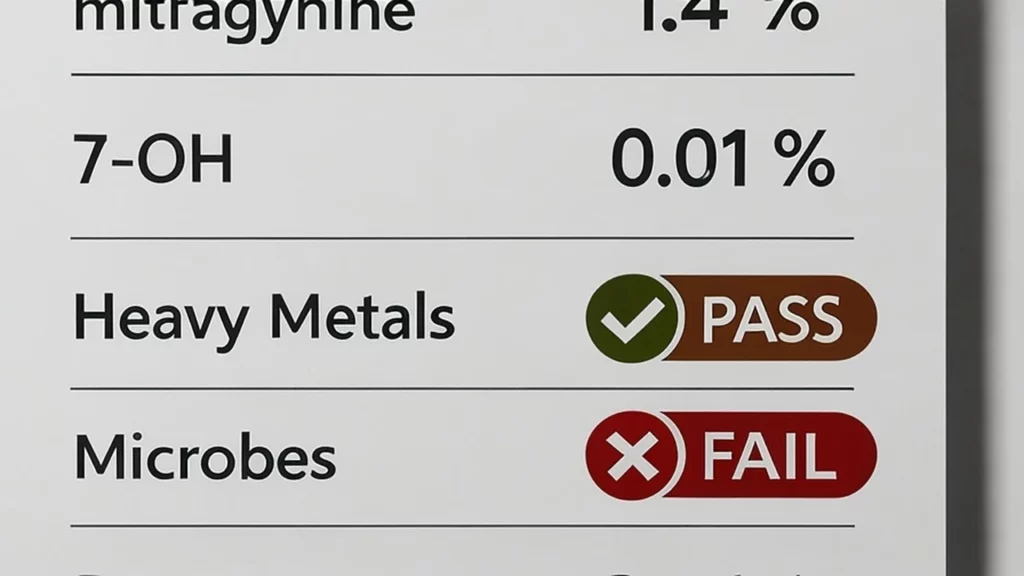
In Canada, kratom is not listed under the Controlled Drugs and Substances Act. This status makes it legal to possess and sell as a natural health product, provided it carries no therapeutic claims. Vendors should follow Good Manufacturing Practices (GMP) and provide third-party lab testing for heavy metals, microbial contamination, and alkaloid content. Certificates of Analysis (COAs) must be available upon request.
United States: FDA Oversight and GMP Programs
The United States classifies kratom as a “Drug of Concern” per the FDA. An ongoing import alert has existed since 2014. Over 1,500 shipments are detained annually at ports for adulteration or undeclared drug claims. The U.S. Pharmacopeia (USP) rejected a 2022 petition to create a kratom monograph due to insufficient safety data. No official USP grade exists. However, the American Kratom Association’s GMP Standards Program has been adopted by over 40 vendors.
Global Benchmarks: Thailand and WHO
In Thailand, kratom was decriminalized in 2021 under the Kratom Act. This law allows cultivation and sale of leaves with less than 0.2 percent 7-hydroxymitragynine. The governing body that oversees kratom now licenses processors and mandates barcode tracking from farm to shelf. The World Health Organization reviewed kratom at the 44th ECDD meeting in 2021. It recommended against international scheduling. The reason cited was “no significant public health risk” at traditional doses. However, it flagged adulteration concerns.
For consumers, these frameworks translate to three practical checkpoints:
- Source verification: Ask for COAs showing mitragynine (0.5 to 2 percent) and 7-OH (less than 0.02 percent).
- Processing transparency: Avoid “enhanced” or “extract” products lacking full alkaloid profiles.
- Regional compliance: Canadian buyers benefit from stricter NHP oversight than U.S. retail.
Understanding these rules does not prove BDNF effects. It ensures any discussion starts with clean, standardized material. This step serves as a prerequisite for consistent biological outcomes.
Frequently Asked Questions
Q: Has any human study directly measured kratom’s effect on BDNF levels?
A: No. No published clinical trial has assayed serum or cerebrospinal fluid BDNF before and after kratom administration. All current claims rely on animal data, cell culture, or indirect biomarkers.
Q: What dose of kratom is most often linked to potential BDNF support?
A: Low to moderate doses (1–5 g of dried leaf) appear in preclinical models and user reports. Higher doses (above 8 g) shift toward sedation and may suppress rather than support neurotrophic pathways.
Q: Can kratom replace prescription antidepressants for BDNF-related benefits?
A: No. Kratom is not an approved medicine. SSRIs and other FDA-approved drugs have robust human BDNF data; kratom does not.
Q: Is kratom legal in Canada for BDNF-related use?
A: Kratom is legal to sell and possess in Canada as a natural health product, but vendors cannot make therapeutic claims, including any mention of BDNF or cognitive effects.
Q: How can I verify the quality of kratom if I want to explore these pathways?
A: Request a third-party Certificate of Analysis (COA) from an ISO-17025 lab. It should list mitragynine (0.5–2 %) and 7-hydroxymitragynine (<0.02 %), plus negative tests for heavy metals and microbes.
Q: Does strain colour (red, green, white) affect potential BDNF interaction?
A: Strain differences reflect alkaloid ratios, not proven BDNF specificity. Red strains are typically higher in 7-hydroxymitragynine (sedative), while white strains lean toward mitragynine (stimulant). No human data isolate BDNF impact by colour.
Q: Are there known drug interactions that could negate BDNF effects?
A: Yes. CYP3A4 inhibitors (e.g., grapefruit juice, certain antibiotics) raise mitragynine levels and risk toxicity. Opioid medications may blunt any indirect BDNF benefit through receptor competition.
Conclusion: The Path Forward for Kratom and BDNF Research
Researchers could employ randomized, placebo-controlled designs with BDNF as a primary endpoint. Neuroimaging (fMRI, PET) would clarify regional activation patterns. Longitudinal cohorts tracking cognitive batteries alongside biomarker panels could establish dose-response relationships. Until such data emerge, discussions of kratom BDNF effects remain hypothesis-driven. Individuals considering kratom should prioritize lab-tested sources, start with minimal effective doses, and monitor personal responses.
The interplay between plant alkaloids and human neurochemistry continues to reveal layered complexities. BDNF represents one node in this network, and kratom’s potential influence, while unproven in people, merits continued scientific attention.
Disclaimer
This blog is intended for informational and educational purposes only and does not constitute medical advice, diagnosis, or treatment. Kratom (Mitragyna speciosa) is not approved by the U.S. Food and Drug Administration (FDA), Health Canada, or any other regulatory authority for any therapeutic use, including effects on brain-derived neurotrophic factor (BDNF), cognitive function, mood, or neurological health. All discussions of potential kratom-BDNF interactions are hypothetical, based on preclinical animal studies, in vitro research, limited observational data, and pharmacological extrapolation.
No controlled human clinical trials have directly measured kratom’s impact on BDNF levels or related brain health outcomes. Individual responses to kratom vary widely due to factors such as dose, strain, preparation, metabolism, and concurrent medications, and self-experimentation carries significant risks. Kratom use has been associated with adverse effects including nausea, constipation, dependence, withdrawal symptoms, liver toxicity, seizures, respiratory depression, and (in rare cases) fatal overdose, particularly when combined with other central nervous system depressants, opioids, or adulterated products.
Pregnant or breastfeeding individuals, those with pre-existing liver, kidney, heart, or psychiatric conditions, and anyone taking prescription medications (especially CYP3A4 substrates/inhibitors) should avoid kratom entirely. Consult a qualified healthcare professional before considering any substance for health-related purposes, and never substitute kratom for evidence-based medical interventions. The authors and publishers assume no liability for any outcomes resulting from the use or misuse of information presented herein.